Abstract
Introduction: Ibrutinib is an oral Bruton's tyrosine kinase (BTK) inhibitor which has transformed the management of several B-cell malignancies, particularly chronic lymphocytic leukemia (CLL). B-cell malignancies inherently confer increased risk of infections due to defects in innate and adaptive immunity. Recent studies have provided conflicting data regarding the risk of infection with ibrutinib therapy, with some reports suggesting an increased risk of infection owing to BTK inhibition while others suggesting decreased risk due to B-cell recovery and humoral reconstitution. We conducted a systematic review and meta-analysis of all phase III randomized controlled trials (RCT) to determine the relative risk of infection associated with the ibrutinib.
Methods: We performed a systematic search of Embase, PubMed, Web of Science, Scopus, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, and Clinical Trials.gov with appropriate keywords through 07/10/18, to find all RCTs comparing ibrutinib with other agents or placebo in patients with B-cell malignancies and also reporting infection as a treatment-emergent adverse event. The search strategy, study selection, data extraction, and analysis were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines. We pooled the point estimates using random effects model of the generic inverse-variance method described by Der Simonian and Laird. Statistical analyses were performed using the Stata/SE 15.1 (StataCorp LP, College Station, TX, USA).
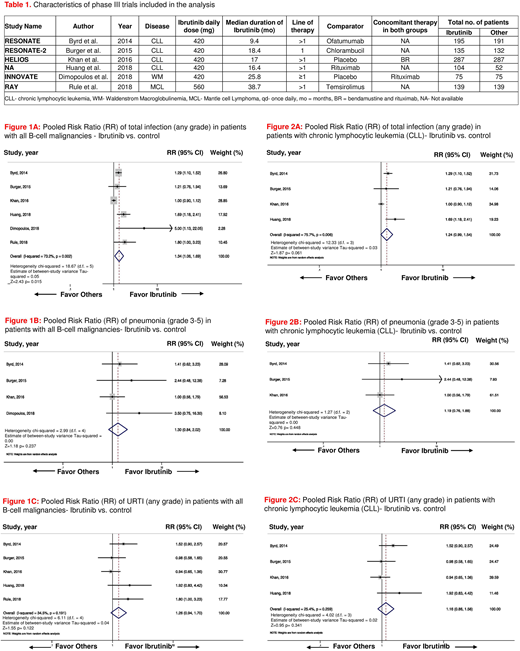
Results: A total of 6 phase III RCTs randomizing 1811 patients (pt; 935 on ibrutinib arms and 876 in the control arms) were included in the final analysis. Four trials were conducted in pts with CLL, one in pts with Waldenstrom macroglobulinemia and the other one in pts with mantle cell lymphoma. Characteristics of these trials are shown in Table 1. In 4 RCTs ibrutinib was compared with an active agent (i.e., ofatumumab, chlorambucil, temsirolimus, and rituximab) and in the other two trials, it was compared with placebo. Ibrutinib was administered as a 1st-line therapy in the RESONATE-2 trial and both as 1st line and in refractory disease in the iNNOVATE trial. The median duration of treatment across studies with ibrutinib was 17.7 months (range 9.4-38.7 months). Ibrutinib was associated with a statistically significant increased incidence of total infections (any grade) in pts with B-cell malignancies [pooled risk ratio (RR) = 1.34, 95% confidence interval (CI): 1.06-1.69, p=0.015, I2=73.2%, fig. 1A]. Pneumonia and upper respiratory tract infection (URTI) were two most commonly reported infection in pts across trials. The incidence of pneumonia (grade 3-5) and URTI (any grade) with ibrutinib was not significantly different from the same in the control arm (pooled RR = 1.30, 95% CI: 0.84-2.02, p=0.237, I2=0.0%, fig. 1B, and pooled RR =1.26, 95%CI: 0.94-1.70, p=0.122, I2=34.5%, fig. 1C respectively). In subgroup analysis of trials (n=4) in pts with CLL only, ibrutinib was associated with a trend towards increased incidence of total infection (any grade) in pts with CLL, but the result was not statistically significant (pooled RR =1.24, 95% CI: 0.99-1.54, p=0.061, I2=75.7%, fig. 2A). No statistically significant difference was noted in the incidence of pneumonia (grade 3-5) and URTI (any grade) in pts with CLL on ibrutinib, as compared to their counterparts in the control arms (pooled RR =1.19, 95%CI: 0.76-1.88, p=0.448, I2=0.0% fig. 2B, and pooled RR =1.16, 95%CI: 0.86-1.56, p=0.341, I2=25.4%, fig. 2C, respectively). No publication bias was observed across all the studies included in final analysis.
Conclusion: In this meta-analysis, ibrutinib was associated with significantly increased risk of infection in pts with B-cell malignancies, whereas this risk was not significantly increased when the analysis was limited to patients with CLL. Future well-designed prospective trials with longer duration of follow up are needed to truly evaluate the association of ibrutinib and infections in pts with B-cell malignancies.
Short:Takeda Oncology: Consultancy. Maiti:Celgene Corporation: Other: Research funding to the institution.
Author notes
Asterisk with author names denotes non-ASH members.